The Genetics of Achromatopsia:
Genetics is about how information is stored and
transmitted between generations.
- John M Smith
All humans are believed to carry variant genes. Infact, each human, on average, carries about six defective genes. This is out of 20,000 to 25,000 genes in the human chromosomes. Genes carry the instruction codes to create proteins or RNA to build the human body. Most of us are unaware of these impaired genes, as they do not create major problems in our life. In achromatopsia several different gene variants can each separately create the condition we call achromatopsia. Not all genetic causes of achromatopsia may have been identified, but the ones discussed in this section account for about 79% of all cases.
There are two main forms of congenital achromatopsia, rod monochromatism and blue cone monochromatism. Each has a different form of inheritance. Rod monochromatism is an autosomal recessive condition and is the most common form of achromatopsia. Rod monochromats usually have more vision loss, more color vision loss, and greater light sensitivity. They can be complete with no color vision or incomplete with traces of color vision.
Blue cone monochromatism is an X-Linked inherited condition. It is an incomplete form of color vision loss with retention of blue cone function. Since blue cones account for only 4% of the cones in the retina, blue cone monochromats still experience most of the same difficulties as the rod monochromats. Blue cone monochromats may as a group have slightly better visual acuity and may retain some color vision in the blue and yellow spectrums.
Rod Monochromatism Genetics
The rod monochromatism is the most common form of achromatopsia. It is inherited as an autosomal recessive condition that requires receiving a gene from each parent. It can be can be caused by more than one gene mutation. So far, mutations or variants of any one of these four genes, CNGA3, CNGB3, GNAT2 and recently PDE6C, have been linked to each causing rod monochromatism. Together, these four genes appear to account for about 79% of cases of rod monochromatism.
from each parent. It can be can be caused by more than one gene mutation. So far, mutations or variants of any one of these four genes, CNGA3, CNGB3, GNAT2 and recently PDE6C, have been linked to each causing rod monochromatism. Together, these four genes appear to account for about 79% of cases of rod monochromatism.
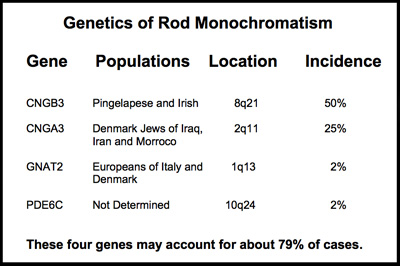
The chart to the right shows the four chomosomes that can carry these gene variants causing rod monochromatism.
Shown on the chart below are the four genes, each independently capable of causing rod monochromatism. They reside on three different chromosomes 1, 2 8, and 10.
These four genes each provide instruction sets for the formation of a protein essential to the normal functioning of the cone receptors. Mutations in any of these genes may impair the cone receptors ability to react to light or process the cone signal.
A recently identified gene, PDE6C, appears to be a possible cause of about 2% of rod monochromatism cases. More studies will be needed to fully understand its role in rod monochromatism ans or progressive cone dystrophies.
CNGB3
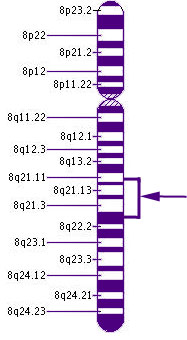 Chromosome 8Here, we see the location on Chromosome 8 of CNGB3, the most frequent cause of rod monochromatism. Chromosome 8 contains about 146 million DNA building blocks.
Chromosome 8Here, we see the location on Chromosome 8 of CNGB3, the most frequent cause of rod monochromatism. Chromosome 8 contains about 146 million DNA building blocks.
CNGB3 stands for cyclic nucleotide gated channel beta 3. It is located on the long arm of chromosome 8. Its cytogenic location is 8q21-q22.
This genetic form is also responsible for the cases of rod monochromatism on the Island of Pingelapese made famous in Oliver Sacks famous book, The Island of the Color Blind. It is believed that a marooned Irish sailor on a British ship may have been the source.
CNGA3
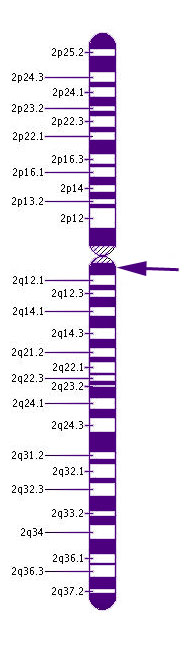 Chromosome 2CNGA3 is responsible for about 25% of cases of RM. This gene may result in either complete or incomplete forms of rod monochromatism. More than 50 different mutations in this gene have been shown to affect color vision.
Chromosome 2CNGA3 is responsible for about 25% of cases of RM. This gene may result in either complete or incomplete forms of rod monochromatism. More than 50 different mutations in this gene have been shown to affect color vision.
This gene is located on chromosome 2 at the cytogenic location 2q11.2. Chromosome 2 has more than than 243 million building blocks of DNA.
CNGA3 protein is part of a family of proteins that form cyclic nucleotide-gate (CNG) channels. CNGA3 is short for cyclic nucleotide gated channel alpha 3. The CNGA3 gene provides the coded instruction sets to make a protein that forms part of an ion channel. Ion channels are openings in the cell membrane that transport electrically charged atoms (ions) into and out of cells. These CNG channels are involved in transmitting signals from the cone receptors. This change in ion transport alters the cone cell's electrical charge and impairs transmission to the brain.
GNAT2
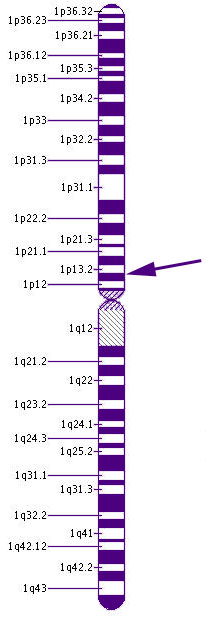 Chromosome 1GNAT2 is the gene that provides the instruction set for part of the creation of t ransducin, a chemical essential for transmitting signals from the cone receptors. When light reaches the photopigments of the cones, the transducin causes a series of chemical reaction that alter the electrical charge of the cell and thus fires a signal to the brain.
Chromosome 1GNAT2 is the gene that provides the instruction set for part of the creation of t ransducin, a chemical essential for transmitting signals from the cone receptors. When light reaches the photopigments of the cones, the transducin causes a series of chemical reaction that alter the electrical charge of the cell and thus fires a signal to the brain.
GNAT2, stand for guanine nucleotide binding protein (G protein), alpha transducing activity polypeptide 2, is located on the short arm of Chromosome 1 at the cytogenic location 1p13.1.
Newly Identified Gene PDE6C
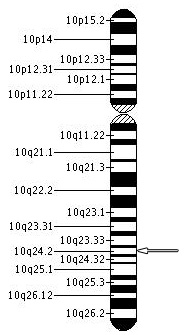 Recent studies using genetic mapping techniques has strongly suggested that the PDE6C gene, phosphodiesterase-6, located on chromosome 10 at 10q24 position may be another genetic cause of achromatopsia or other cone dystrophies. Variants in PDE6C gene appear to result in early onset cone disorders. PDE6C plays a critical role in the cone photo transduction process.
Recent studies using genetic mapping techniques has strongly suggested that the PDE6C gene, phosphodiesterase-6, located on chromosome 10 at 10q24 position may be another genetic cause of achromatopsia or other cone dystrophies. Variants in PDE6C gene appear to result in early onset cone disorders. PDE6C plays a critical role in the cone photo transduction process.
Autosomal Recessive Inheritance of Rod Monochromatism
Rod monochromatism is an autosomal recessive condition. Acquiring rod monochromatism requires that we have inherited a “abnormal” gene from each parent. If we inherit one “normal” and one “affected” gene, we would be a carrier, because in this situation one “normal” gene alone is enough to instruct our body how to produce the appropriate chemicals for cone development or function. If, however, we inherit a gene causing rod monochromatism from each parent, we then lack the instruction code to make the necessary proteins required for functional cone cells and would have rod monochromatism.
Rod monochromatism: Risks of having an affected child.
The risk of a rod monochromat having an affected child is extremely low since those with the condition and those who are carriers are rare in society. However, every child of a rod monochromat will be a carrier of the condition.
The typical inheritance of rod monochromatism (RM) involves two asymptomatic parent carriers, each providing one gene. With each birth, there is a 25% risk of RM being expressed. Statistical risks, however, are more accurate in large numbers, not in a small group of siblings. The exact outcome in each family cannot be predicted. Just as in tossing a coin we might get four tails in a row or four heads in a row or some combinations in between. Therefore, other brothers and sisters of an achromat may also acquire the condition.
Except for the following two very rare situations, rod monochromats do not have offspring with rod monochromatism. In the extremely rare case of two individuals with the same form of rod monochromatism having children, 100% of offsprings would have the condition. The other rare option would be a rod monochromat having children with a carrier of the same type of RM. All offspring then would either have the condition or be carriers. In this case, there is a 50% risk of having RM with each birth. Our doctors have never observed a case in which a patient with an autosomal recessive rod monochromatism has produced a child with rod monochromatism.
Carriers of rod monochromatism are asymptomatic, but it has been suggested that these carriers may have mild color vision defects themselves.
Blue Cone Monochromatism Genetics
Blue cone monochromatism is a form of incomplete achromatopsia. Blue cone monochromatism is inherited on the X chromosome. It has an incidence in men of 1 in 50,000 to 1 in 100,000 births and may be as low as 1 in 10,000,000,000 births in females. It is a rare X
monochromatism is inherited on the X chromosome. It has an incidence in men of 1 in 50,000 to 1 in 100,000 births and may be as low as 1 in 10,000,000,000 births in females. It is a rare X The X Chromosome-chromosome inherited disorder. It is characterized by loss of functioning of the red and green cones. Patients retain blue cones and rods. It is strongly associated with the gene array of Xq28 on the X chromosome.
The X Chromosome-chromosome inherited disorder. It is characterized by loss of functioning of the red and green cones. Patients retain blue cones and rods. It is strongly associated with the gene array of Xq28 on the X chromosome.
Recently two genes have been assigned that together could disrupt the red and green cone functions, OPN1LW and OPN1MW. These are located in the locus of Xq28 on chromosome X.
OPN1LW
OPN1LW stands for red cone pigment (opsin 1, long-wave-sensitive). It is a gene on the X chromosome, which is crucial to the creation of the red cones photopigment, the chemical that lets the cone react to long wavelengths like red.
OPN1MW
OPN1MW stands for opsin 1 (cone pigments), medium-wave-sensitive. It is a gene on the X chromosome, which is crucial for the green cones to function. This gene provides the instruction set to create the green cone photopigment, the chemical that reacts to medium wavelengths like green light. We still have much to learn about these genes and others that may be involved.
Blue Cone Monochromatism Inheritance Issues
Females have two X chromosome. Men have one X chromosome and one Y chromosome. Thus, if they inherit an affected gene on the X chromosome, they have no possibility of receiving a normal gene to provide the appropriate genetic instructions and thus override the abnormal gene.
Females have two X chromosomes. Thus, even if they inherit one affected gene they have the high likelihood of having a non-affected gene on the other X chromosome that can override the problem. In rare cases, the one “non-affected” gene may not be enough to prevent the woman from developing BCM. This is way we must say it is technically possible for a female to have Blue cone monochromatism, but essentially it is a condition of men.
Female carriers of blue cone monochromatism have normal appearance to the retina, are asymptomatic, but may demonstrate slightly abnormal cone function on an electroretinogram (ERG) and abnormalities on eye movement recording.
Passing on the BCM Gene
It is important to understand that men determine the sex of all children by passing either their X or Y chromosome. If a man passes on an X chromosome, the child is a female. If the man passes on a Y chromosome, the child is a male. Thus men can receive their X chromosome where the gene variant exists for BCM only from their mother and can only pass this X chromosome gene mutation on through their daughters.
All daughters would receive the gene, but nearly always they would be carriers only. Male grandchildren may inherit BCM from carrier mothers if she passes the one X chromosome with the mutation. Since she has two X chromosomes, a mother with only one copy of the gene mutation has a 50% risk of passing the gene on to both sons and daughter. Males receiving the affected gene will all have the disease, but females receiving the affected gene will nearly always be carriers only.
A typical family history of BCM may include male siblings with BCM and a grandfather and/or male cousin(s) on the mother's side. It could show again in grandsons from a daughter of the Blue Cone Monochromat, but not in sons of the affected person. It is not impossible for females to have the condition, but it is extremely rare.
Carver Laboratories Genetic Tests
Genetic Tests for Rod Monochromatism mutations of CNGB3 (Exon10) and CNGA3 (Exon 8) are now available through the John and Marcia Carver Nonprofit Genetic Testing Laboratory at the University of Iowa.
Genetic Treatments
See othe next section on the potential to treat achromatopsia with genetic treatments.
